
What is chelated calcium and what isn’t?
As we discover more about what chelated calcium seems to do and how different it is from traditional calcium sources I thought I would try to create a simple explanation about what chelated calcium is and what it isn’t. This article doesn't discuss what chelated calcium does for horses. Please see the "Missing Link" and "Journey" articles for more about that. Or visit our YouTube channel:
Our Non-oxalate research: https://www.youtube.com/watch?v=SMjc2a_hCkY&t=31s
Our oxalate research: https://www.youtube.com/watch?v=YhpX_5jmA38&t=572s
This article has been written for the Facebook group Chelated Calcium Stories: Australia. So in places it refers to competitors and environmental conditions (high oxalate pastures) specific to Australia. However the content is relevant to horse owners around the world as chelated calcium helps horses everywhere.
Unfortunately there are people in the equine nutrition industry who either don’t understand it or refuse to look at how it is different to other chelates or who simply deliberately muddy the waters. I doubt I will be able to simplify it as much as we would all like but here goes:
To understand all of this we are going to have to explore some rather confusing words like organic and inorganic which mean one thing to a farmer and something completely different to a chemist. Unfortunately nutrition gets stuck between these two and has to grapple with both uses of these words.
What does a farmer or a food buying consumer mean by organic?
Organic food is basically food that has been produced without the use of modern pesticides or fertilisers. Certain ingredients for use in organic farming can also be used in animal nutrition so you may see supplements and or feeds that claim to be organic or that use organic ingredients.
What does a chemist or biologist mean by organic?
To a chemist organic molecules are molecules containing mostly multiple carbon atoms. In these organic molecules the carbons are generally joined together in chains or ring structures. These are mostly quite large molecules and are the building blocks of life like amino acids, sugars, fats etc. Many of these are the molecules that are able to chelate with minerals like calcium, magnesium, iron, zinc etc etc.
Not all carbon containing molecules are organic. Where the carbon isn’t joined to another carbon it doesn’t constitute a chemists idea of organic. So that means that molecules like calcium carbonate or sulphate or chloride or phoshate are NOT organic. Confused yet?
Deliberate or unintentional confusion
There are firms in Australia that blur these two definitions. By buying certified organic (farming sense) calcium carbonate (which is available from algal sources in the pristine seas around New Zealand or Ireland) they describe their product as organic. But they imply it is organic in the chemists’ sense and, completely incorrectly, claim it is chelated. It isn’t – it is just calcium carbonate – chemically like limestone or chalk but more expensive.
I have even seen it blended with flour or sugar (a source of starch/sugars). Starch is a chemist’s organic molecule. But mixing starch with calcium carbonate doesn’t make a chelate. It has to undergo a chemical bonding process. The main culprit for this is Julie Cook and I can say that with confidence as we have tested her product (sent directly from the customer to the laboratory unopened and untampered with) using very sophisticated analytical techniques.
Diagrammatic representation of these different molecules and what happens when they dissolve
I will use some diagrams from different sources so please bear with me that they are not consistent. Let’s start with a simple inorganic molecule like calcium carbonate (limestone or chalk or cuttlefish bone or the shells of molluscs):
Chemical formula: CaCO3
Structure:
Let me try to explain this. Firstly note that the three oxygen molecules are attached directly to the carbon to make the “carbonate” entity. The calcium has donated two electrons to the carbonate and, as electrons are negatively charged that is why you see the 2- sign by the carbonate and the 2+ by the calcium.
When in solid form this structure is closely associated and also forms a mass with other identical molecules to look something like this:
You will be perfectly familiar with this as an off-white powder or rock (limestone and chalk) or stalactites and stalagmites in caves or the shells of molluscs like oysters and snails.
When calcium carbonate dissolves it separates into the two entities (called ions) and because the electrons stay with the carbonate the electric charges remain with each ion.
Calcium carbonate cannot dissolve without splitting up like this.
Contrast this with Calcium gluconate (formula C12H22CaO14). Here the carbons are joined in a chain (organic chemists are lazy and they don’t like putting “C” all over the place so they represent the carbon chain as an angled line where every kink/angle represents a carbon atom). So the two gluconate molecules in this diagram each has 6 carbon atoms:
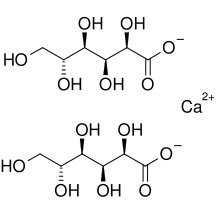
Calcium gluconate is held together by the electrical attraction between the calcium and the two sugar molecules. This is a very stable bond (and some other calcium chelates are even stronger). They are strong enough that these molecules can dissolve in water without dissociating. And that means that the electrical charges are all held within the one big molecule which is therefore an uncharged entity. That will become important later on.
Drawing chemical molecules like this is always a huge simplification. Just to add another level of complication let me show you another chelate (calcium citrate) where there are three calciums trapped between two citrate molecules. To complicate it further water binds to this too (even when in the solid form as below):
Once dissolved this diagram is probably a better representation with two of the calciums truly chelated and the third bridging the two citrate molecules together:
I’m afraid that we have competitors who are in denial about the solubility of chelated calcium molecules in body fluids and who still insist that it dissociates and gives up its calcium ions. So here is a diagram that shows how all the calcium in the body is distributed:
Let me try to explain this:
1. 99% of our calcium is in our teeth and bones
2. The other one percent is either in the cells or in the fluids that bath the cells (which includes blood). Let’s look at that in more detail:
a. The cytosol is the main fluid inside the cells that isn’t in organelles. All animals and plants invest heavily in pumping calcium out of the cytostolic fluid because that is where we locate the calcium receptors that we use to switch the cell off and on. It must be as close to calcium free as possible.
b. The main cell organelles that contain a lot of calcium are the mitochondria and the endoplasmic reticulum (ER). The purpose of these stores of calcium ions is to enable calcium signalling to go on all over the cell. So when these stores pump calcium into the cytoplasm the cell is activated. It is our belief that chelated calcium has a role in removing calcium ions from the cytosol to the ER and mitochondria through channels called VDACs – just throw me a few million dollars for the research required to prove or disprove that theory :).
c. The extra cellular fluids and the blood also contain calcium ions (that can be pumped into the cytosol to switch the cells on). That is about 55% of the calcium in the blood – though some books would put this as low as 47% and we know that one of our trial horses finished best British horse at the Endurance World Champs (12th overall) and his ionised calcium (iCa) was just 46% of his total blood calcium.
d. There is a large amount of calcium bound to protein (mostly albumin) in the blood and extracellular fluids. This appears to have no function and doesn’t even seem to measured by the calcium homeostatic process. Measuring blood calcium without also measuring protein and albumin levels can be very deceptive. We have often seen horses with low total blood calcium diagnosed as hypocalcemic when actually it was their very low blood protein that caused the result. Their ionised blood calcium was perfectly normal. Sadly few equine vets have been trained to look at iCa. For some reason bird vets picked up on this a couple of decades ago.
e. That leaves the smallest component “Complexed Calcium”. These are molecules that dissolve without dissociating. The eagle eyed amongst you will see that this includes both inorganic (calcium bicarbonate and calcium phosphate) and organic molecules (calcium citrate and lactate – though this can also include gluconate, amino acid chelates, and our enemy – calcium oxalate).
Our research strongly suggests that this complexed calcium is measured by the homeostatic system. That would be a pretty difficult trick compared to measuring iCa so nature would not have developed a system like that without having a purpose. And that suggests that chelated calcium has a job to do.
Hopefully you can begin to see why nobody has studied chelated calcium.
1. Firstly it only represents a tiny proportion of the calcium in the body.
2. It has no electric charge and ALL the techniques used to study calcium signalling and other ion transport involve studying those charged ions. That means it is invisible to currently used techniques.
3. There are loads of different organic and inorganic molecules in this “complexed” group. How would you identify the ones that matter from the ones that don’t?
A number of years ago we arranged a meeting with the calcium expert at Bristol University (his boss is a neighbour of mine which is the only reason we got access). He said “If Britain allocated all of its science research budget to trying to answer your question (what chelated calcium does) then, in ten years time – we probably still wouldn’t have an answer.” And that was from a man who had developed one of the most important techniques for measuring the movement of calcium ions across cell membranes. So guys – if you want scientific proof of my theories here – go somewhere else :).
In all the above discussion I have used the term chelate the way nutritionists do. In these molecules every calcium is bonded to two organic molecules. A puritanical chemists would probably argue that a chelated should have two bonds to the same molecule.
What about calcium oxalate?
Calcium oxalate is a true chelate. But it is a much smaller molecule than the “good chelates” we have discussed up to now.
Formula: CaC2O4
Structure:
Here you can see that the electrical charges that hold the chelate together bind the calcium to a single oxalate molecule.
Pretty well all the members of this group will be familiar with the damage that calcium oxalate does to their horses. I hope they are beginning to recognise that the explanations they have been given about how it interferes are, at best questionable. We have an article that discusses this point on our Australian website (https://equifeast-australia.com/the-oxalate-debate/).
What we don’t know is why or how calcium oxalate causes its problems. The idea that it causes calcium deficiency isn’t supported by the trials we have run or the experience of many in this Facebook group. All the achievements we make involve feeding less calcium not more.
It could be a matter of molecular size. Our bird business hints that calcium lactate (a smallish molecule) is less effective than the larger gluconate, citrate or amino acid chelates. This may relate to the size of molecule that VDACs transport into the mitochondria – or that may have nothing to do with it. Obviously calcium oxalate is a very small molecule.
Do we need a blend of chelate and inorganic calcium?
I am only addressing this here because when Jenquine were told by Ad Standards to stop exaggerating the amount of chelated calcium in their products they changed promotional message and now argue that there is a blend of the two that makes for the perfect effect. Many of you have tried that blend with no success and I would argue that amount of chelated calcium they use is so small as to be insignificant. I was very careful when I reported their marketing to Ad Standards not to question the efficacy of the Bone Formula Forte. But the Industry Jury assembled by Ad Standards observed that there were a number of players in the market with very small chelated calcium additions (we had supplied the summary of our analysis of a number of competitors who have made chelated calcium content claims but with similarly tiny amounts in their formulations). The lawyers that made up the jury wanted to know if those blended products actually had an efficacy – a good question that shows that they really did understand what they were being asked to investigate. I am sure that, because Jenquine claimed to have “Clinical Trials”, they expected they would be presented with loads of data supporting the Jenquine formulation. That data could have been presented confidentially. But Jenquine didn’t offer anything at all. Not even any veterinary anecdotes. So much for clinical trials.
I am still open-minded. So far we haven’t found any cases where supplementing with inorganic calcium, magnesium or phosphorus alongside chelated calcium has been needed. Our lick formulation does contain a small amount of DCP. It was the only thing we could find to reduce the palatability enough to prevent the horses just bogging into the lick. It seems to be working very well on the horses that get it (mostly working horses that are between musters or mares and foals in large paddocks not hand fed every day).
I hope this article helps a little and doesn’t muddy the waters even further.
Malcolm Green – Technical Director, EquiFeast.

