Article by Malcolm Green - Technology Director EquiFeast Australia.
27th December 2019
From a very wet England I recognise that many people in Australia are currently suffering terribly from drought, bushfires and a horrendous heatwave. When I last lived full-time in Australia the fires were bad - but clearly nowhere near as desperate as they are now. In the early 1980s one of the products in my marketing portfolio was the sticky phosphate chemicals used to water bomb bushfires. Our sales team called on fire departments in Tassie, Victoria and South Australia. Fires were so minor further north we didn't even regard NSW and QLD as significant markets. How things in the environment change. Our thoughts are with you all. Please keep you and your livestock safe.
I don't want to appear insensitive at this time but I have been surprised by the interest in this Jenquine advertising topic on the Aussie Horse Health and Nutrition Group and feel some matters merit comment, correction and putting ‘the other point of view’.
If you only read one little piece of this article make it point number 9 - click here
The Australian Consumer Law and the Australian Association of National Advertisers Code requires advertisers to avoid misleading consumers and businesses. After nearly three years trying to avoid this and attempting to get Jenquine to engage in discussions with us, EquiFeast reluctantly reported Jenquine to Ad Standards for its misleading web site, product leaflet, videos and social media posts.
Please be clear:
* Our sole objective is to ensure that consumers and vets are completely clear that even though Jenquine's marketing made their Bone Formula Forte product look much like our BREAK FREE, that is not the case.
# Consumers and vets are entitled to expect products to be accurately described in such a way that they can differentiate between them.
# This is all about the difference in the product make up and therefore the expectation that the two products will perform differently in various situations.
* We have never questioned the efficacy of Jenquine's products. Our complaint is 100% about the misleading way they promote their product and how they make it look like ours when it is completely different.
* We have never made personal comments about any individual from Jenquine. This is a strong contrast to Jenquine whose initial reply to Ad Standards contained two and a half pages attacking myself and EquiFeast. The Industry Jury (three eminent lawyers appointed by Ad Standards) determined this was completely irrelevant to their investigation.
Here are our three complaints taken directly from our letter to Ad Standards:
- Jenquine’s marketing is constantly invoking their use of Chelated Calcium implying to vets and consumers that their product contains a substantial amount of this ingredient as well as claiming its contribution to be critical to the performance of their product. Yet our analysis shows that only 1.65% of the calcium in their main product (Bone Formula Forte) is chelated. This contrasts with 100% of the calcium in our product (BREAK FREE) being chelated.
The Industry Jury appointed by Ad Standards split this complaint into two that they called "Content Representations" and "Efficacy Representations".
They upheld our complaint.
- It is our belief that, in order for consumers to be able to make an informed comparison between products, Jenquine are obliged to quantify their products’ content of a nutrient their marketing claims is crucial (Chelated Calcium). They consistently refuse to do so both on their web site (see below) and on social media. The amount of chelated calcium in their product is very low.
This complaint was not upheld by the Ad Standards Industry Jury who determined that an advertiser is entitled to keep the exact amount of an active ingredient secret. This is a contrast with the rules in the UK but personally I am more than happy with this ruling because there is little point in developing new technology if it has to be given freely to a competitor.
- Jenquine claim their calcium is chelated to the amino acid methionine. Our analysis says they use Calcium MHA which is not an amino acid.
The Industry Jury appointed by Ad Standards upheld our complaint.
As a result of this ruling Jenquine have made major changes to their website (including removing all bar two of their FAQs referring to chelated calcium), they have withdrawn their product leaflet entirely (its content was dominated by chelated calcium and didn't ever mention any other forms of calcium at all) and (although only after some months of pressing by Ad Standards) they have removed all references to Methionine on their website because their product doesn't contain any. But their recent social media posts have countered many of the findings that Ad Standards made demonstrating that they regard the regulator and the regulation process as irrelevant. Furthermore this suggests they also regard consumers’ and veterinary surgeons’ "right to know" as irrelevant.
A recent thread on Aussie Horse Health and Nutrition (Facebook) contains a large number of statements from Jenquine - mostly by their Customer Service and Marketing person (Kristie Morris). I would like to deal with many of these points one by one. I am also going to take this opportunity to make comments about some of the other posts on this thread. I will accompany my comments with snips from the thread and from some other documents such as our submission to Ad Standards and the Ad Standards report itself.
Let's start by putting up the original post as the rest makes more sense if we read that:
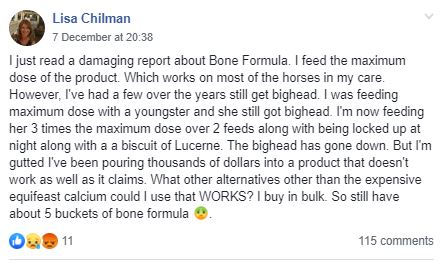
Plenty of people made comments that are genuine attempts to answer Lisa's question and then this appears from Kristie Morris: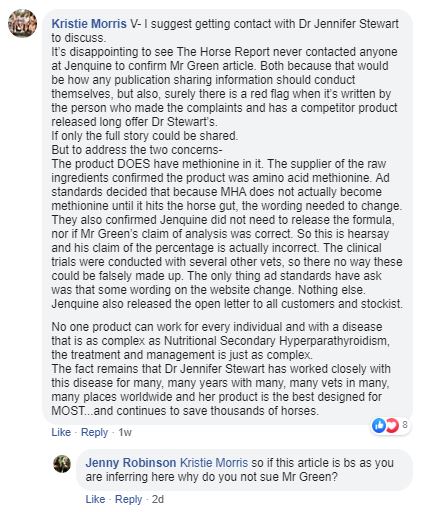
1. The Horse Report didn’t need to contact Jenquine before publishing. The Ad Standards Industry Jury report is in the public domain for anyone to see and I imagine they read it. There is the advertiser statement in the report (section 6) so they already had Jenquine’s official view. Here is the link to the full Ad Standards report:
2. I would dispute that our product was launched "long after Dr Stewart's". Yes their original Bone Formula was launched (I think) in 2008. We ran our field trials in 2012 and launched late that year. In July 2016 the Jenquine brand was created and Bone Formula Forte was launched. The changes to the formulation were drastic:
- They removed all the phosphorus
- They removed all the magnesium
- Their marketing emphasis on chelated calcium was escalated dramatically to the point that the Ad Standards industry Jury wrote this:

When I point out to readers that EquiFeast's BREAK FREE product, as sold since 2012, contains no phosphorus or magnesium and that 100% of its calcium is chelated it becomes clear that, in terms of this technology, Jenquine is the follower not the leader. And, of course, sadly they have neither the amount nor the form of chelated calcium in the product that they imply - and that is crucial. They are dressing in our clothes but they are covering up a body that is not at all like ours. I'll leave you to decide if that is misleading or deceptive.
3. Kristie's statement that their product DOES (her emphasis) contain methionine is nothing short of bizarre and, to me, highlights that Jenquine have absolutely no respect for the truth. Our analysis was clear that they use MHA which is not an amino acid let alone methionine. This was the only part of our complaint where Jenquine offered any evidence to Advertising Standards, as is required by the process. They obtained permission to provide a letter from their supplier (Novus) that would not be made public or shown to me – which is perfectly fine. So I don't know exactly what it said. But it is clear that it didn't convince the Industry Jury that MHA is methionine.
This may not worry you. The body turns MHA into methionine - though why anyone should supplement an oxalate affected horse with methionine is not clear, nor is it ever mentioned in Jenquine's material that methionine is itself a functional component except as the chelating ligand.
But it may worry you if you don't want to feed your horse a chemical that nature never invented. In fact MHA was created by Monsanto in 1956. Novus is the company spun off from Monsanto and still has its head office in St Louis, MO like their original parent.
Novus markets this technology for pigs, poultry and cattle. They have no advice or data for its use in horses. Again that may or may not worry you.
Jenquine had implied their product was pharmaceutical grade - nothing Novus makes is Pharma grade.
As I say - this may not worry you. But it clearly worries Jenquine because they have been very reluctant to apply the regulator's ruling in this matter.
Here is the chart from our laboratory analysis. It is the result of pretty sophisticated spectrographic technology. The peaks from MHA are clearly marked:
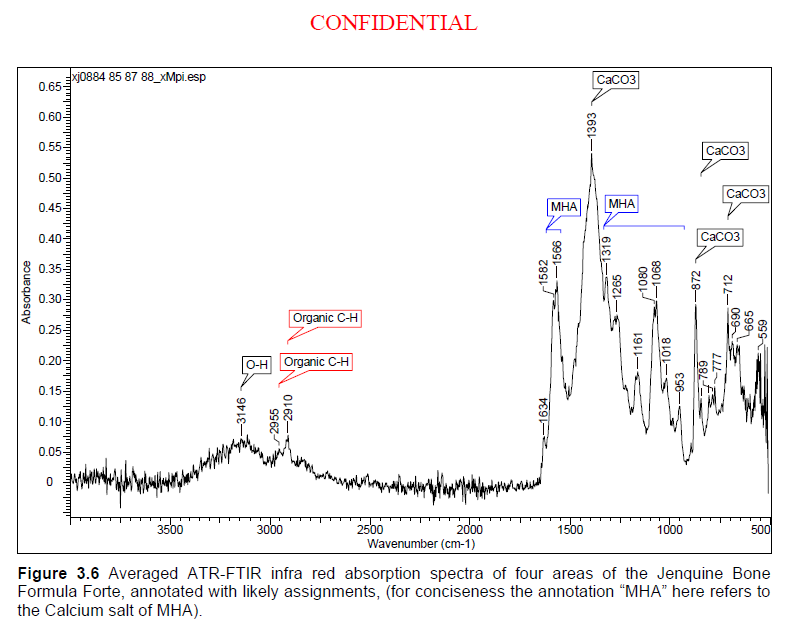
4. The idea that our analysis is nothing more than "hearsay" is equally bizarre. Our analysis was conducted in a world leading laboratory. A whole unopened 5 kg pail of Jenquine's product was bought by a third party and shipped direct to the laboratory and photographed as it was unpacked and opened to confirm we had not tampered with it. The only redaction in the analytical report is the name and address of the customer who kindly purchased and shipped the sample. You can read the whole analytical report here.
The Industry Jury accepted the report, in my opinion, for two reasons:
* It is a very sound piece of work by a world leader in the field
* Jenquine offered absolutely no evidence to contradict it – the Ad Standards process seeks evidence from defendants in all cases. My conclusion from that is that, had they asked their manufacturer to provide evidence, it would simply have confirmed the analysis.
Here is what the Industry Jury wrote – and note lawyers don't lightly throw around words like "... as a matter of fact":

It is a complete snub to Ad Standards and to horse owners to claim the analysis is inaccurate or hearsay when Jenquine were specifically asked to provide their evidence months ago by the regulator. The AANA code requires that advertisers have substantiation of their claims readily available.
5. Kristie has mentioned clinical trials. What clinical trials? Again the regulator asked for evidence of efficacy and, I believe, evidence that their small amount of chelated calcium was effective. I imagine they expected to be bombarded with the full "clinical trial" report. Jenquine offered absolutely no evidence at all. Probably because they have none. See lower down this page for confirmation of this.
6. Kristie wrote "The only thing ad standards have ask was that some wording on the website change." Hmmm! I'll leave you to judge if this is reasonable. We pointed out to the Industry Jury that there were seven FAQs referring to chelated calcium. Their current website only has two. That seems like a fairly drastic reduction. They have completely removed their product leaflet from the website (it's emphasis on chelated calcium was huge), they have removed mentions of chelated calcium from their product description and accompanying graphics and replaced it with "organic calcium" and they reluctantly and slowly removed all references to methionine.
Just below Kristie's comment this appeared - Good question Jenny :)
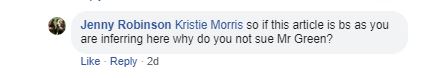
Summary of points 1 - 6 - I seem to have six major complaints about just 200 odd words of Kristie's post - Wow!
7. This next section also deserves some comment:
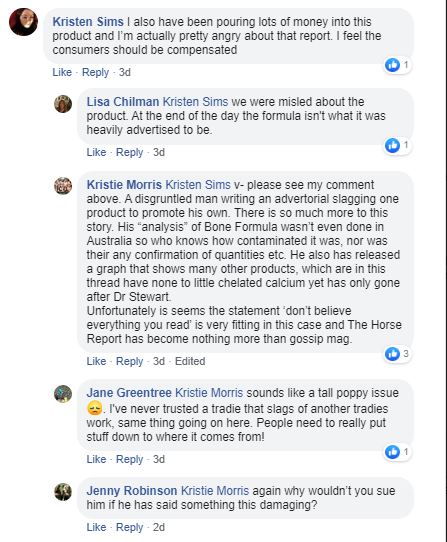
Maybe I am a disgruntled man. Although I am an Australian I currently live in the UK. I was asked to come to Australia to see if our unique approach to calcium might help with this problem called Big Head (in my opinion a bad name for a much more insidious disease). I had absolutely no idea so, before anything else, we set up field trials. That involved finding a manufacturer to make small quantities of product, finding trial sites (we selected Murwillumbah and Blackall - a very long way from anywhere). I spent thousands of dollars visiting those sites on numerous occasions, inspecting the animals, discussing the tests we wanted done and, with the help of Trevor Wozencroft in Queensland and fabulous support from the Gribbles veterinary lab (who arranged for some blood tests to be done in human hospitals in Melbourne and Sydney) we got the trials done. Imagine my amazement when I later discovered that no other manufacturer or academic has conducted any trials on their technology when they live just down the road from thousands of affected horses.
The whole industry here is relying on the work published in 1981 by McKenzie et al. There is nothing wrong with that work though, in light of our experience, I would question the underlying assumptions they made. Their trials were too short for symptoms to be an issue and, of course, nobody was thinking about chelates back then so they shine no light on the technology we are discussing here.
I never claim our trials are science - they were field trials. In fact I go much further than that - I passionately complain about firms that say they have science when they don't. Here is an example of the second slide in almost all of my presentations. It makes the point that we are a technology industry of trials not scientific experiments or clinical trials. This one was from Melbourne's Equitana in 2014:

But at least our field trials exist - when it seems very clear that Jenquine's "clinical trials" do not.
Am I slagging off another product? I haven't questioned its efficacy at all (though the Industry Jury did). All I am asking is that Jenquine (and others) are clear and honest about their own products. Then you will see that our technology is in a class of its own. That doesn't make it better, it makes it different. And that means it works differently and in different circumstances. It will fail in different circumstances too. Consumers have a legal right to that information and some of those who have been misled have rightly made their feelings known in this Facebook thread.
8. This next little exchange highlights that different products will work and fail in different circumstances. At EquiFeast we are proud of our track record working with clients to try to better understand those circumstances. Indeed we won a UK National award for the way we leveraged our customer relationships into our R&D programme. Of course clients have to be prepared to work with us and, not unreasonably many are not.
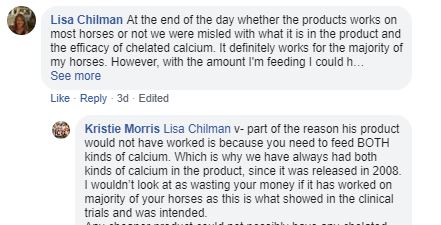
Kristie's comment here about having to have both kinds of calcium is clearly not founded in any sort of data. The AANA code requires advertisers to hold data for claims like that. I believe Jenquine don't. I can be confident of that for two reasons:
1. They presented no data or trials information to Ad Standards when it was requested
2. Further down this thread they say they don't have any!
9. This next point is a real killer. Read Kristie's first sentence very carefully.
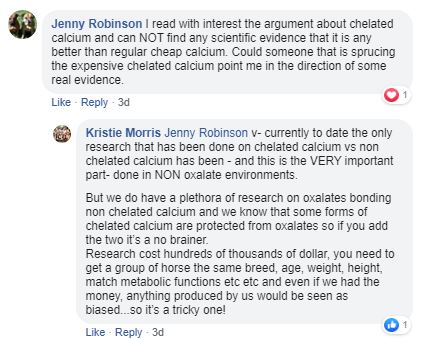
So here it is. Absolute confirmation that Jenquine have no research on chelated vs non chelated calcium in oxalate environments. So no clinical trials after all. So how have they determined what is their "optimum blend"? This is a staggering statement in the light of everything else that Jenquine claims! At the very best my guess is that Jenquine have a small number of veterinary anecdotes. No controls, no placebos no "clinical trials". The vets involved are not named and there is nothing published in any form at all.
And then the fact is, Kristie's statement isn't true. There is our research. Limited though it is, it is real and was conducted on pure oxalate pastures (buffel and setaria in Blackall and Murwillumbah respectively). We selected horses specifically with existing symptoms of Big Head (by definition serious cases) and they all had to be having traditional calcium supplements while their disease developed. The field trial protocol involved taking away the existing calcium, phosphorus and magnesium supplements and feeding our chelated calcium with a few trace minerals added so the diets didn't change with respect to selenium, copper, zinc etc.. All the horses on our trial got better and showed dramatic improvements in their blood chemistry as well as their symptoms. This isn't science because we didn't have controls or placebos but our trialists and their horses are all identifiable and real and our work is published in video form for anyone to see and criticise.
It is the success of these field trials combined with our subsequent field experience that has caused us to question many of the basic assumptions that influence the way oxalate poisoning has been treated for many years. You can read about our ideas here.
10. The exchange below again raises the unsubstantiated claim that oxalate affected horses need both chelated and inorganic supplements. This is a complete contradiction of our field trial experience. But we have just seen that Jenquine have absolutely no trial data that supports that theory at all. They were asked for this by the Industry Jury and they failed to provide it. Similarly their point about the chelated calcium in their product equating to 500 grams a day of inorganic calcium (carbonate/phosphate) is completely bizarre. How on earth is 0.25 grams of chelated calcium supposed to achieve that? Their argument goes that even if all the calcium in the feed and inorganic supplements is bound by oxalate there is still enough chelated calcium in their product to satisfy the horse's needs. That requires 20 or more grams of chelated calcium. Not 0.25 grams. Evidence of claims like this are required by the AANA code.
For me these statements are a problem because they devalue the whole chelated calcium technology. Support a technology with claims that make no sense and credibility is damaged. As the only people with real experience of chelated calcium we can't reconcile Jenquine's explanation of how it works with either what we see it do in the real world or the highly stable nature of chelated calcium (which doesn't seem to donate its calcium ion to the system). Here is a repeat link to our theory. Note the word theory.
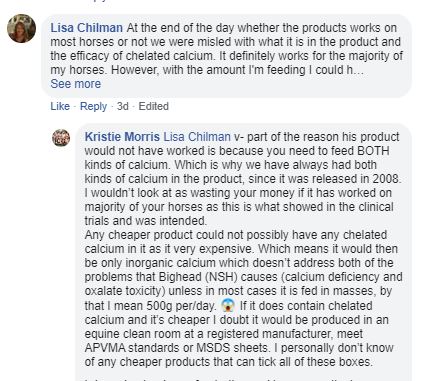
11. When they overstated their chelated calcium back in 2016 they started down a dangerous cul-de-sac. They have now been backed into a corner and seem to be digging themselves a big hole to fall into. Look at this bizarre statement:

According to this the offending video was removed 12 months ago. That means about December 2018. Our complaint letter went to Ad Standards in April 2019 and contained this link to the video:

We didn't send them a screenshot so the link must have worked because the Industry Jury report carries a complete transcript of the video. Jenquine hadn't taken it down at all!
They still have videos available talking about chelated calcium and how they ran clinical trials using a "special chelated calcium formula". Dr Stewart describes in this video how they measured PTH levels when feeding this formula. She also notes that the Royal North Shore Hospital no longer offers that service so PTH tests are not available. Well the RNSH stopped offering that service in 2013 - I know because we couldn't get them done. So is it likely that Jenquine was working on their new improved 2016 formulation back in 2012 and 2013? And if they were, why didn't they send this information to Ad Standards?
You can watch this video here. It is very convincing but is it true?:
https://www.youtube.com/watch?v=lc7eZxRvLq0&list=PL9ykGQQMpQhgxWtNQRkqHGLTCk71cuX2P&index=5
Towards the end of the Ad Standards process Jenquine removed nearly all of their calcium and oxalate related videos. All bar the one on Bone Formula Forte have been restored. Suggesting they feel the dust has settled and they no longer have to comply with the ruling.
Summary
Tragically Jenquine still seems to think it is OK to put forward a whole host of what are, at best, theories as if they were scientifically proven science. These are what the Industry Jury refers to as "unconditional statements". Such statements have to be supported by rigorous evidence - which Jenquine hasn't provided. They seem to claim to have conducted "clinical trials" but then admitted in this thread that isn't true. Yet they still have videos on YouTube that talk about this mythical research.
In many ways Jenquine’s behaviour is not the most important issue. What matters is that the Ad Standards report and all of our supporting information shows beyond doubt that Jenquine's Bone Formula Forte is a limestone and lime product with a sprinkling of fairy dust (chelated calcium). The calcium in EquiFeast's BREAK FREE is, in contrast, 100% chelated and it delivers 24 times as much chelated calcium for roughly 5 times the price. Clearly these two products are in a completely different classes. And yes - we have put our own product (and a number of others) through the same rigorous analysis as Jenquine's. BREAK FREE's claimed analysis is clearly confirmed!
If you are angered by Jenquine’s behaviour you can complain to Ad Standards yourself. It is free and confidential for consumers to do so:
https://adstandards.com.au/lodge-complaint
Thank you for taking the time to read this.
Malcolm Green
Technical Director - EquiFeast Australia
https://www.facebook.com/MalcolmGreenEquiFeast
email: malcolm@equifeast.com
